Dive Brief:
- The U.S. Food and Drug Administration on Tuesday proposed requiring front-of-package nutrition labeling on most packaged foods to give “accessible, at-a-glance information to help consumers quickly and easily identify how foods can be part of a healthy diet.”
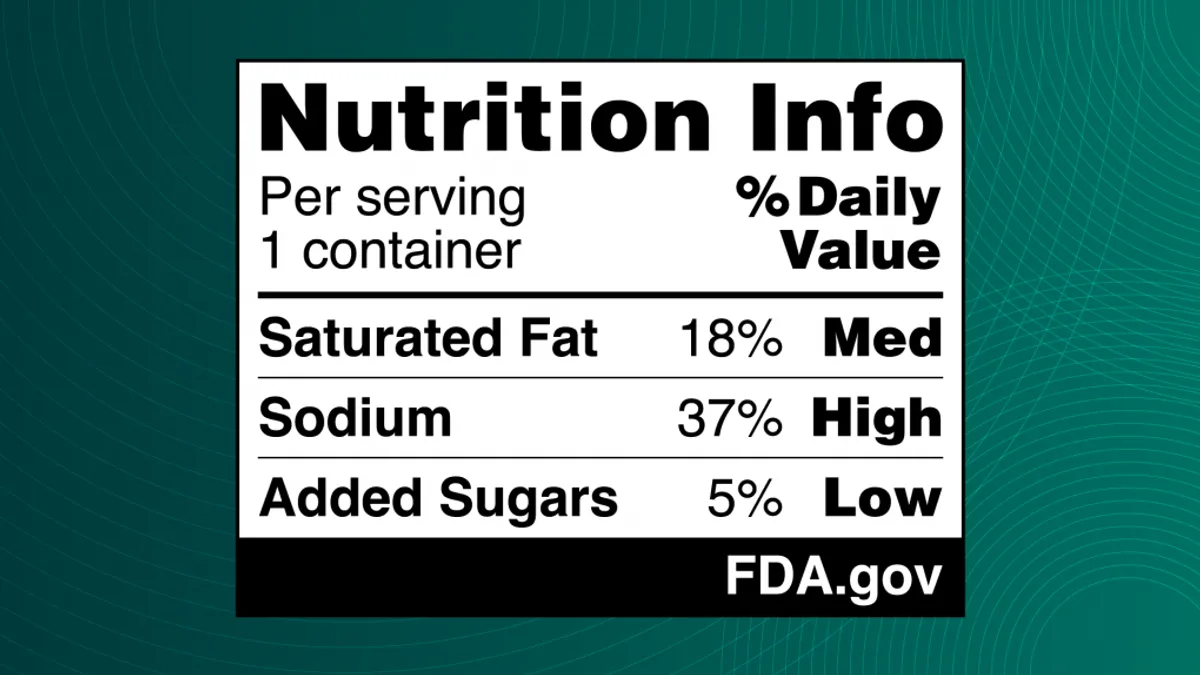
- The proposed nutrition label, called the Nutrition Info box, would let shoppers know if a single serving has low, medium or high amounts of saturated fat, sodium and added sugars.
- FMI – The Food Industry Association said in a Tuesday statement that it is still reviewing the proposed mandate but worries it would oversimplify a product’s dietary makeup, displace important information like date labels and require costly redesigns of most food packaging labels.
Dive Insight:
The FDA said the proposed front-of-pack label would complement the Nutrition Facts label already required on most packaged foods. The agency noted that current federal dietary recommendations advise consumers to limit their consumption of saturated fat, sodium and added sugars.
“While many consumers use and benefit from the Nutrition Facts label, regular use of the label is lower among some segments of the population,” the FDA said, adding that other countries have already successfully implemented front-of-package nutrition labeling.
The agency noted that manufacturers could voluntarily add calorie information to the front of the food package.
The proposed label would be required to appear in the upper third of the principal display panel — a placement that FMI claims would result in packaging label redesigns and could move other key information, like date labels, to another part of the package.
FMI Chief Public Policy Officer Jennifer Hatcher also said the trade group was disappointed the FDA did not select the Facts Up Front, a voluntary labeling program it helped co-create, as part of the proposed rule. This program’s labeling design highlights product information like calories, saturated fat, sodium, added sugars and beneficial nutrients such as fiber and calcium.
“We also believe that reducing a food’s entire dietary contribution to whether it is low, medium or high in saturated fat, sodium, and added sugar is overly simplistic and will not help educate consumers on how to improve their overall dietary pattern,” Hatcher said. “However, we are pleased to see that the agency allowed for the inclusion of calories alongside the FOP label.”
FMI also praised the FDA’s decision to keep the label black and white.
The FDA is receiving comments on the proposed rule until May 16. If the rule goes into effect, businesses with $10 million or more in annual food sales would have three years to come into compliance, while those below that threshold would have four years.
Prior to proposing the rule, the FDA conducted a literature review, two sets of focus group testing and a peer-reviewed experimental study on consumer reactions to different front-of-pack nutrition labels.